9.86 – 9.33 billion years ago
More on stardust and us.
Looking at the abundance of different elements in the universe, we get the following:
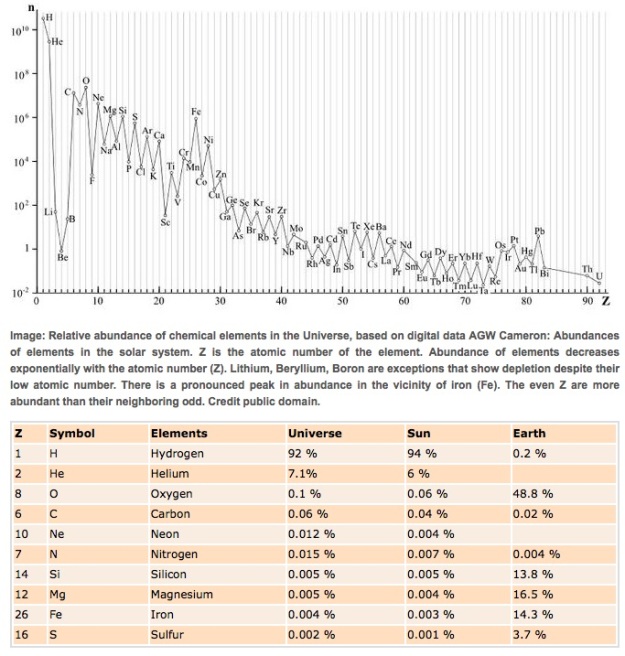
Note that the vertical scale is logarithmic, so there is vastly more hydrogen and helium in the universe than any other element. As noted in the last post, all the elements except hydrogen and helium were formed after the Big Bang, spewed out by supernovas and the collisions of neutron stars. In general, heavy elements are less abundant because it takes more steps to produce heavy elements than light ones. But the curve is not smooth. The lightest elements after hydrogen and helium (lithium, beryllium, boron) are relatively rare, because they get used up in the nucleosynthesis of heavier elements. And there is a saw tooth pattern in the chart, because nucleosynthesis favors atoms with even numbers of protons. So we get lots of oxygen, magnesium, silicon, and iron, the main constituents of our planet. Lots of carbon too. Finally, iron (Fe) is more than 1000 times more abundant than might be expected based on a smooth curve. Iron nuclei are especially stable because binding energy, the energy that would be required to take the nucleus apart into its constituent protons and neutrons, reaches a maximum with iron. Here’s the famous curve of curve of binding energy (nucleons are protons and neutrons):
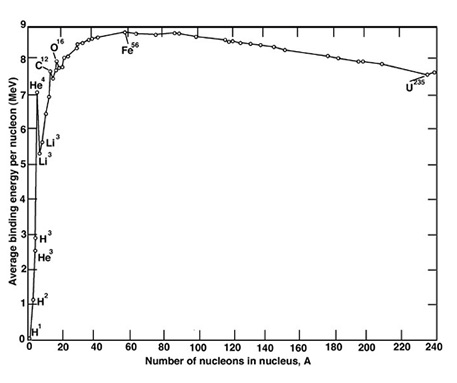
An implication of this curve is that if you can split a really heavy nucleus, of Uranium-235 say, into smaller nuclei (but still heavier than iron), you will release energy equal to the vertical difference between U-235 and its lighter fission products (not shown) on the vertical scale. This is lots of energy, way more than you get from breaking or forming molecular bonds in ordinary chemical reactions. And if you can fuse two light nuclei, of hydrogen say, into a larger nucleus, you can get even more energy. When we split uranium, we are recovering some of the energy that a star put into synthesizing elements heavier than iron, before or as it went supernova. When we fuse hydrogen, we are extracting energy left over from the Big Bang that no star got around to releasing. (This doesn’t violate the Law of Conservation of Energy, because the negative gravitational potential energy of the universe cancels the positive energy represented by the matter. So the total energy of the universe is zero.)
Starting to figure this all out was part of a scientific revolution that made physics in 1950 look very different from physics in 1900. The new physics resolved a paradox in the study of prehistory. Geologists were pretty confident, based on rates of sedimentation, that the Earth had supported complex life for hundreds of millions of years. But physicists couldn’t see how the sun could have kept shining for so long. The geologists were right about deep time; it took new physics to understand that the sun got its energy from fusing hydrogen to helium (via some intermediate steps).
As the scientific revolution in atomic physics was picking up steam, it was natural to assume that it would be followed by a revolution in technology. After all, earlier scientific revolutions in the understanding of masses and gases, atoms and molecules, and electrons and electromagnetism, had been followed by momentous innovations in technology: the steam engine, artificial fertilizers, electrification, radio, to name just a few. But in some ways, the Atomic Age hasn’t lived up to early expectations. The atom bomb brought an earlier end to the Second World War, but didn’t change winners and losers. The bomb was never used again in war, and it’s a matter for debate how much the atom bomb and the hydrogen bomb changed the course of the Cold War. Nuclear energy now generates a modest 11% of the world’s electricity (although this number had better go up in the future if we’re serious about curbing carbon dioxide emissions). And a lot of ambitious early proposals for harnessing the atom never got anywhere. Project Plowshare envisioned using nuclear explosions for enormous civil engineering projects, digging new caves, canals, and harbors. Even more audacious was Project Orion, which developed plans for a rocket propelled by nuclear explosions. Some versions of Orion could have carried scores of people and enormous payloads throughout the solar system. Freeman Dyson, a physicist who worked on the project, said “Our motto was ‘Mars by 1965, Saturn by 1970.’”
On the purely technical side these plans were feasible. There were concerns about fallout, but the problems were not insurmountable. Nevertheless both Plowshare and Orion were cancelled. Regarding Orion, Dyson said “… this is the first time in modern history that a major expansion of human technology has been suppressed for political reasons.” The history of the Atomic Age and its missed opportunities is one more refutation of pure technological determinism. How or even whether a new technology is exploited depends on social institutions, politics, and cultural values.
